Description
【GS-441524 MECHANISM】
GS-441524 is s small molecule nucleoside analog that undergoes intracellular phosphorylation under the action of cellular kinases in vivo to produce active triphosphate metabolites (NTPs) that can compete with natural nucleotides in vivo, inhibits RNA polymerase, and interferes with cat infection RNA replication virus, thereby preventing the synthesis of feline infectious peritonitis virus
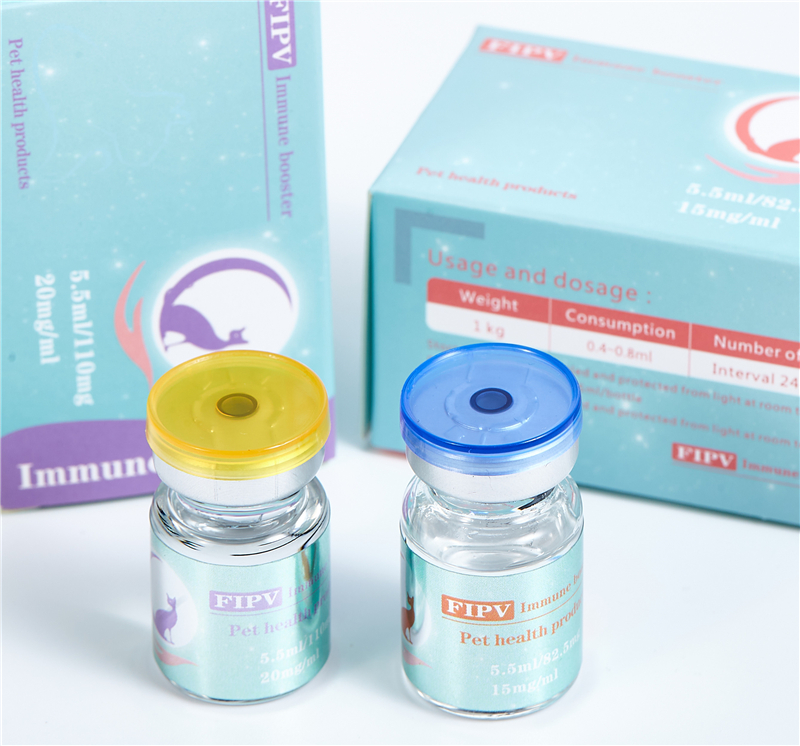
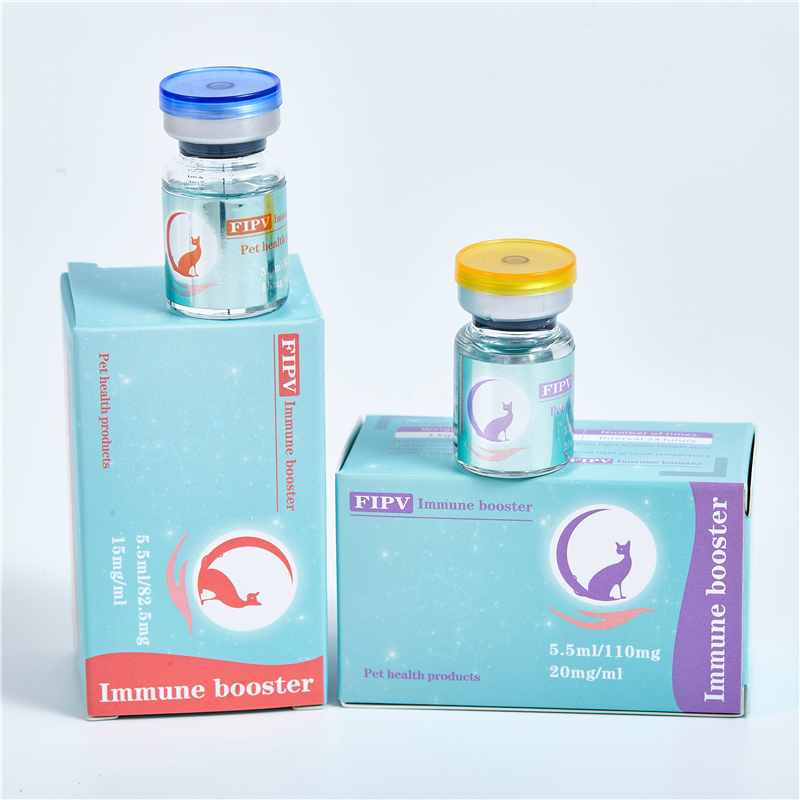
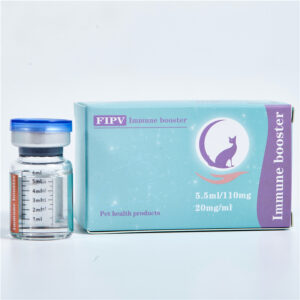
【Specification】15mg/mL, 20mg/mL

【Usage】2.5-5.0 mg/kg.sc.q.24h. requires an experienced operator to avoid injecting the drug
to the same part of the dermis or subcutaneous layer.
【preservation】room temperature, avoid direct sunlight.
【course of treatment】usually after 5 days of use, symptoms are relieved, but due to the absence of more clinical data to indicate more the drug can be discontinued for a long time, and the medication time is decided by the doctor and the owner in consultation according to individual differences
【Description】
This nucleoside is an essential building block to the synthesis of Remdesivir triphosphate (1355149-45-9) and Remdesivir (1809249-37-3). Nucleoside derivate is used in the treatment of Feline infectious peritonitis (FIP). This fatal disease affects cats and is caused by a virus from a group of coronavirus. Remdesivir is a nucleoside prodrug that metabolizes into GS-441524 which is phosphorylated to Remdesivir triphosphate.
【Experiment】
Four of the 31 cats that presented with severe disease died or were euthanized within 2-5 days and a fifth cat after 26 days. The 26 remaining cats completed the planned 12 weeks or more of treatment(GS-441524). Eighteen of these 26 cats remain healthy at the time of publication after one round of treatment, while eight others suffered disease relapses within 3-84 days. Six of the relapses were non-neurological and two neurological. Three of the eight relapsing cats were treated again at the same dosage, while five cats had the dosage increased from 2.0 to 4.0 mg/kg q24h. The five cats treated a second time at the higher dosage, including one with neurological disease, responded well and also remain healthy . However, one of the three cats re-treated at the original lower dosage relapsed with neurological disease and was euthanized, while the two remaining cats responded favorably but relapsed a second time. These two cats were successfully treated a third time at the higher dosage, producing 25 long-time survivors. One of the 25 successfully treated cats was subsequently euthanized due to presumably unrelated heart disease, while 24 remain healthy.